Design solutions
TDR Implementation Research Toolkit
This toolkit provides deeper learning on implementation research, helping people learn a standard process that can lead to results that can be compared across regions and countries. It is designed to...
Implementation Research in Health: A Practical Guide
Implementation issues arise as a result of a range of factors including ‘real world’ contextual factors that are either overlooked or not captured by other research disciplines. Implementation researc...
A system-wide approach to analysing efficiency across health programmes
Health programmes are able to target health interventions for specific diseases or populations, and historically, countries have relied heavily on them to deliver priority services. In low- and middle...
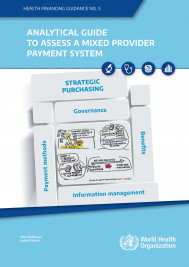
Analytical guide to assess a mixed provider payment system
In nearly all countries, several payment methods co-exist and constitute a mixed provider payment system (MPPS). Providers are paid by several payment methods and are faced with several incentives tha...
Conceptual Background and Case Studies – Introduction to EVIPNet Europe
EVIPNet is a knowledge translation network established by WHO. EVIPNet envisions a world in which policy-makers and other stakeholders in low- and middle-income countries use the best available resear...
Facilitator's guide: using research in the EVIPNet framework
This Guide provides complete instructions and materials needed to conduct a workshop in which participants are guided towards (i) acquiring, assessing, adapting and applying evidence that is relevan...
Lessons from the Health Evidence Network (HEN): Lessons learned about delivering an evidence synthesis service for policy-makers
HEN is an information service for public health decision-makers in the WHO European Region, in action since 2003 and initiated and coordinated by the WHO Regional Office for Europe. HEN supports publi...
OneHealth Tool
The OneHealth Tool is a software tool designed to inform national and subnational strategic health planning in low- and middle-income countries.While many costing tools take a narrow disease-specific...
Options for best practice clinical guideline development in Ukraine: policy brief
This policy brief summarizes the results of this study and describes best practice structures, processes and methods for guideline development in Ukraine. Key messages include the requirement for inve...
Sound Choices: Enhancing capacity for evidence-informed health policy
This Guide addresses a mismatch between what is known about how to respond to particular health problems in poor economies and what is actually done about them. It focuses on one cause of the problems...
SUPPORT Tools for evidence-informed health Policymaking (STP) 1: What is evidence-informed policymaking?
This article is part of a series written for people responsible for making decisions about health policies and programmes and for those who support these decision makers.In this article, the following...
SUPPORT Tools for evidence-informed health Policymaking (STP) 2: Improving how your organisation supports the use of research evidence to inform policymaking
This article is part of a series written for people responsible for making decisions about health policies and programmes and for those who support these decision makers.In this article, ways of organ...
Systems Thinking for Health Systems Strengthening
In the previous decades, little was understood about what works in health systems strengthening and how researchers and policymakers can apply it. So, although there was general agreement for the need...
WHO Cervical Cancer Prevention and Control Costing (C4P) Tool
The Department of Immunization, Vaccines and Biologicals (IVB) in WHO has developed a generic costing and planning tool for cervical cancer prevention and control. The WHO Cervical Cancer Prevention a...
The WHO-INTEGRATE evidence to decision framework version 1.0: integrating WHO norms and values and a complexity perspective
Evidence-to-decision (EtD) frameworks intend to ensure that all criteria of relevance to a health decision are systematically considered. This paper, part of a series commissioned by the WHO, reports...
SPECTRUM Generalized Cost-Effectiveness Analysis
SPECTRUM Generalized Cost-Effectiveness Analysis (GCEA) is a tool developed to facilitate country-level cost-effectiveness analysis of a wide range of health interventions. The tool is populated with...
Tools for making good data visualizations: the art of charting
Data visualization is a collection of methods that use visual representations to explore, make sense of and communicate quantitative data. It allows trends and patterns in quantitative data to be seen...
Climate change and health: a tool to estimate health and adaptation costs
Adaptation is one of the approaches to prevent or reduce some of the effects caused by climate change, including health effects. To support ministries planning for climate change adaptation measures,...
Costing tool for estimating the cost of interventions to improve hand hygiene in domestic settings
A critical building block to achieving the global goal of universal hand hygiene by 2030 is adequate levels of funding. Understanding the costs of implementing hand hygiene plans is an essential precu...
Step-by-step guide to conducting a cross-programmatic efficiency analysis
The World Health Organization (WHO) has developed a diagnostic approach to enable countries to look across health programmes that are part of their health system to detect “cross-programmatic” ineffic...
E4As guide for advancing health and sustainable development: resources and tools for policy development and implementation
Achieving the Sustainable Development Goals (SDGs) requires working in transformative ways. This guide is a compilation of tools and resources for advancing health and sustainable development in cou...
National Immunization Strategy costing application (NIS.COST)
During the past two decades, the comprehensive multi-year planning (cMYP) process has been the cornerstone of strategic planning for immunization as part of the Global Vaccine Action Plan (2011-2020)....
Support tool to strengthen health information systems: guidance for health information system assessment and strategy development
This tool is an update of the 2015 Support tool to assess health information systems and develop and strengthen health information strategies. Both the global General Programme of Work and the Europea...
Climate change mitigation, air quality and health (CLIMAQ-H) tool
The Climate change mitigation, air quality and health (CLIMAQ-H) calculation tool allows quantification of the physical and economic consequences for human health achieved through improvements in coun...
Assessing financing, education, management and policy context for strategic planning of human resources for health
This document contains a method for assessing the financial, educational and management systems and policy context, essential for strategic planning and policy development for human resources for heal...
Mental health investment case: a guidance note
WHO and UNDP prepared this note to provide a structured approach for making national investment cases for mental health. The note complements the Non-communicable disease prevention and control: a gui...
Implementing Health in All Policies: a pilot toolkit
To support countries implementing HiAP approaches, this WHO document – Implementing Health in All Policies: A pilot toolkit - has been prepared to support HiAP practice across regions, and inform the...
Evidence briefs for policy. Using the integrated knowledge translation approach. Guiding manual
Evidence Briefs for Policy (EBPs) are a relatively new, innovative approach to packaging research evidence for policy-makers; however, they are already the most widely used tool. EBPs are prepared by...
GRADE Handbook
The GRADE handbook describes the process of rating the quality of the best available evidence and developing health care recommendations following the approach proposed by the Grading of Recommendatio...
Evidence synthesis for health policy and systems: a methods guide
This methods Guide explores various ways to address the challenges of synthesizing evidence from HPSR including methods and tools to conduct and promote the uptake of evidence synthesis for health pol...
Strengthening national evidence-informed guideline programs. A tool for adapting and implementing guidelines in the Americas
The purpose of this document is to present policy-oriented and methodological strategies for developing and/or strengthening national guideline programs, focusing on the adaptation of evidence-informe...
WHO handbook for guideline development
A WHO guideline is any document developed by the World Health Organization containing recommendations for clinical practice or public health policy. A recommendation tells the intended end-user of the...
A resource for developing an evidence synthesis report for policy-making
HEN – the Health Evidence Network – is an information service for public health decision-makers in the WHO European Region, in action since 2003 and initiated and coordinated by the WHO Regional Offic...
Guide to qualitative evidence synthesis: evidence-informed policy-making using research in the EVIPNET framework
This guide aims to support country efforts to generate evidence, including qualitative evidence. It summarizes what a qualitative evidence synthesis is, and how it can contribute to the evidence-infor...
Health Technology Assessment Toolbox for Emerging Settings: Best Practices and Recommendations
This toolbox is focused on the use of health technology assessment (HTA) in emerging settings. Its primary audience is&nbs...
Making GRADE the Irresistible Choice authoring and publication platform (MAGICapp)
MAGIC (Making GRADE the Irresistible Choice) is a non-profit foundation with the goal of increasing value and reducing waste in healthcare through a digital and trustworthy evidence ecosystem. MAGICap...
Rapid reviews to strengthen health policy and systems: a practical guide
Policy-makers require valid evidence to support time-sensitive decisions regarding the coverage, quality, efficiency, and equity of health systems. Systematic reviews and other types of evidence synth...
SUPPORT Tools for evidence-informed health Policymaking (STP) 5: Using research evidence to frame options to address a problem
This article is part of a series written for people responsible for making decisions about health policies and programmes and for those who support these decision makers.Policymakers and those support...
A Guide for Evidence-Informed Decision-Making, Including in Health Emergencies
This guide aims to bring together the most recent thinking in the EIDM field and to present this in a format that is accessible to a wide audience of EIDM practitioners. It builds on, and contextuali...
Estonian Handbook for Guidelines Development
Clinical practice guidelines are generally accepted as an important tool for improving the quality of clinical care provided by health professionals, as well providing guidance to ensure the quality u...
Institutionalizing health technology assessment mechanisms: a how to guide
As countries face financing constraints in achieving their universal health coverage goals, they must ensure that available resources are spent more efficiently. The institutionalization of healt...
SUPPORT Tools for evidence-informed Policymaking in health (STP) 11: Finding and using evidence about local conditions
This article is part of a series written for people responsible for making decisions about health policies and programmes and for those who support these decision makers.Evidence about local condition...
SUPPORT Tools for evidence-informed health Policymaking (STP) 7: Finding systematic reviews
This article is part of a series written for people responsible for making decisions about health policies and programmes and for those who support these decision makers.Systematic reviews are increas...
SURE Guides for Preparing and Using Evidence-Based Policy Briefs 4. Deciding on and describing policy options
SURE Guide 4 is part of the SURE Guides for Preparing and Using Evidence-Based Policy Briefs. The SURE Guides are intended for those people responsible for preparing and supporting the use of policy b...
COVID-19 Vaccine Introduction and deployment Costing tool (CVIC tool)
The CVIC tool supports credible COVID-19 vaccination costing to facilitate a dialogue with stakeholders, while maintaining sensitivity to protect essential health services.The CVIC tool provides a str...
SUPPORT Tools for evidence-informed health Policymaking (STP) 10: Taking equity into consideration when assessing the findings of a systematic review
This article is part of a series written for people responsible for making decisions about health policies and programmes and for those who support these decision makers.This article addresses conside...
SUPPORT Tools for evidence-informed health Policymaking (STP) 17: Dealing with insufficient research evidence
This article is part of a series written for people responsible for making decisions about health policies and programmes and for those who support these decision makers.In this article, the issue of...
SUPPORT Tools for evidence-informed health Policymaking (STP) 12: Finding and using research evidence about resource use and costs
This article is part of a series written for people responsible for making decisions about health policies and programmes and for those who support these decision makers.This article addresses conside...
Developing national action plans on transport, health and environment: a step-by-step manual for policy-makers and planners
A national transport, health and environment action plan (NTHEAP) is a key tool and mechanism for developing sustainable and healthy transport in a country. NTHEAPs provide a comprehensive and inter...
Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews
Our objective was to develop an instrument to assess the methodological quality of systematic reviews, building upon previous tools, empirical evidence and expert consensus.A 37-item assessment tool w...
Applying GRADE-CERQual to qualitative evidence synthesis findings: introduction to the series
The GRADE-CERQual (‘Confidence in the Evidence from Reviews of Qualitative research’) aims to assess the degree of confidence of the findings from systematic reviews of qualitative research (or qualit...
The Appraisal of Guidelines for Research & Evaluation Instrument II (AGREE II)
The Appraisal of Guidelines for REsearch & Evaluation (AGREE) Instrument was developed to address the issue of variability in guideline quality. To that end, the AGREE instrument is a tool that as...
SUPPORT Tools for evidence-informed health Policymaking (STP) 8: Deciding how much confidence to place in a systematic review
This article is part of a series written for people responsible for making decisions about health policies and programmes and for those who support these decision makers.The reliability of systematic...
SUPPORT Tools for evidence-informed health Policymaking (STP) 16: Using research evidence in balancing the pros and cons of policies
This article is part of a series written for people responsible for making decisions about health policies and programmes and for those who support these decision makers.In this article, the use of ev...
AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both
The number of published systematic reviews of studies of healthcare interventions has increased rapidly and these are used extensively for clinical and policy decisions. Systematic reviews are subject...
SUPPORT Tools for evidence-informed health Policymaking (STP) 9: Assessing the applicability of the findings of a systematic review
This article is part of a series written for people responsible for making decisions about health policies and programmes and for those who support these decision makers.This article suggests question...
Selection of essential medicines at country level: Using the WHO model list of essential medicines to update a national essential medicines list
Since 1977, WHO has been working with countries to design the package of essential medicines as an integral component of treatment within the continuum of care, developing and disseminating the Model...
SUPPORT Tools for evidence-informed health Policymaking (STP) 13: Preparing and using policy briefs to support evidence-informed policymaking
This article is part of a series written for people responsible for making decisions about health policies and programmes and for those who support these decision makers.Policy briefs are a relatively...
SURE Guides for Preparing and Using Evidence-Based Policy Briefs 1. Getting started
SURE Guide 1 is part of the SURE Guides for Preparing and Using Evidence-Based Policy Briefs. The SURE Guides are intended for those people responsible for preparing and supporting the use of policy b...